RefleXion Highlights Clinical Study Results for Future Prostate Cancer Treatment
June 26, 2023
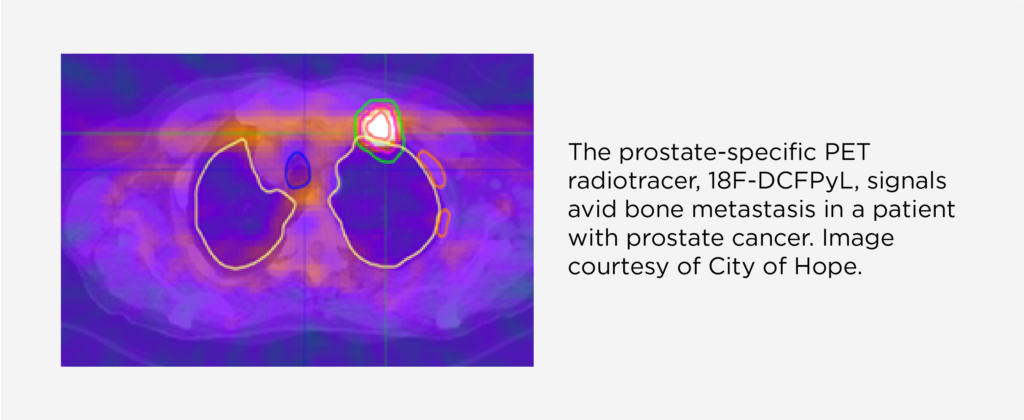
City of Hope researchers establish foundation to expand SCINTIX therapy using a prostate cancer-specific PET radiotracer
HAYWARD, California, June 26, 20223 – RefleXion Medical, Inc., a therapeutic oncology company, today announced that results of a prospective investigator-initiated clinical imaging study conducted on its X1 platform by City of Hope using positron emission tomography (PET) were presented on June 24 during an oral session at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting in Chicago. The study results serve as the foundation for evaluating the use of RefleXion’s SCINTIX™ biology-guided radiotherapy with a prostate-specific PET radiotracer for controlling external-beam radiotherapy delivery to prostate cancer tumor targets. City of Hope, one of the largest cancer research and treatment organizations in the U.S., is among the first in the nation to adopt this new radiotherapy technology that has the potential to change the way metastatic cancer patients are treated.
Recently cleared by the U.S. Food and Drug Administration (FDA), SCINTIX technology is the first and only cancer therapy that uses each cancer’s unique biology to autonomously determine where to deliver radiotherapy, second-by-second, during the actual cancer treatment to indicated solid tumors of any stage. SCINTIX therapy uses signals produced by a PET radiotracer interacting with cancer cells to control delivery of external-beam radiotherapy to tumor targets.
The prostate-specific PET radiotracer used in the presented study – 18F-DCFPyL (PyL) – binds to prostate-specific membrane antigen (PSMA), a protein that is expressed in significantly elevated amounts by prostate cancer cells. Also recently approved by the FDA for diagnosing and staging prostate cancer, PyL can accurately and precisely pinpoint tumors in both the prostate and in other body areas where the cancer may have spread or metastasized.
“It is well established that PyL exquisitely detects tumors present in patients with prostate cancer, but targeting and treating those tumors can be challenging using existing radiotherapy approaches. SCINTIX therapy could overcome these barriers, and our study results support continued exploration of leveraging PyL’s precision to expand SCINTIX therapy to patients with prostate cancer.”
Jeffrey Wong, M.D.
Principal Study Investigator at City of Hope
“It is well established that PyL exquisitely detects tumors present in patients with prostate cancer, but targeting and treating those tumors can be challenging using existing radiotherapy approaches,” said Jeffrey Wong, M.D., professor of the Department of Radiation Oncology and the Department of Immunology and Theranostics at City of Hope, and principal investigator of the RefleXion-supported PyL imaging study. “SCINTIX therapy could overcome these barriers, and our study results support continued exploration of leveraging PyL’s precision to expand SCINTIX therapy to patients with prostate cancer.”
The prospective PyL imaging study established that tumors arising from prostate cancer could be visualized on the RefleXion® X1 platform using signals from PyL consistent with PyL diagnostic imaging studies, and that SCINTIX treatment plans could be generated using these data. PSMA-directed SCINTIX treatment plans also met conventional radiotherapy organ dose constraints, suggesting the ability to spare nearby organs and other healthy tissue from potentially damaging radiation. SCINTIX therapy is currently cleared for use with 18F fludeoxyglucose (FDG), a common PET radiotracer, to treat primary and metastatic tumors in the lung and bone.
“We look forward to offering FDG-directed SCINTIX therapy to our patients in the next several weeks,” said Terence Williams, M.D., Ph.D., professor and chair of City of Hope’s Department of Radiation Oncology. “As early collaborators in evaluating SCINTIX technology, it is gratifying to see research and clinical development efforts already advancing it toward another patient population in great need of improved radiotherapy approaches using the well-characterized benefits of PyL.”
The presentation entitled “A Prospective Pilot Study of the RefleXion X1 PET-CT Subsystem Imaging Performance with [18F]-DCFPyL PSMA in Patients with Prostate Cancer” was part of oral session SS10 “Focus on Clinical Studies – New Insights.”
About RefleXion Medical
RefleXion is a privately held therapeutic oncology company located in Hayward, Calif., commercializing SCINTIX biology-guided radiotherapy, a novel therapy that uses a single radiotracer injection to transform cancer cells into real-time biological beacons to control external-beam radiotherapy delivery to multiple tumors. Granted Breakthrough Device designation for lung tumors and De Novo marketing authorization by the FDA, SCINTIX therapy is indicated for use in lung and bone tumors arising from either primary lung and bone cancers or resulting from metastases by other primary cancers. RefleXion has co-development and co-commercialization agreements with Lantheus and Telix for their PSMA-targeted PET radiotracers as SCINTIX therapy bioguides for prostate cancer applications. The RefleXion X1 is also cleared for conventional image-guided radiotherapy for solid tumors located anywhere in the body.
Contacts
RefleXion
Amy Cook
acook@reflexion.com
925-200-2125
City of Hope
Katherine Ramirez
katramirez@coh.org
626-678-4163