SCINTIX Therapy
Science fiction becomes reality, and a new day dawns for cancer care
March 20, 2023
It sounds like the stuff of science fiction: a technology that can detect the biological activity of cancer cells in the body and direct targeted beams of energy to destroy tumors in real-time. That vision is now a reality, and with it, a new day has dawned for cancer care.
The milestone moment occurred in February with the announcement that RefleXion’s SCINTIX™ biology-guided radiotherapy (BgRT) has received clearance for marketing by the U.S. Food and Drug Administration (FDA). The clearance means that we can now take the next step in our company’s evolution, fulfilling a vision that began with our founding 15 years ago.
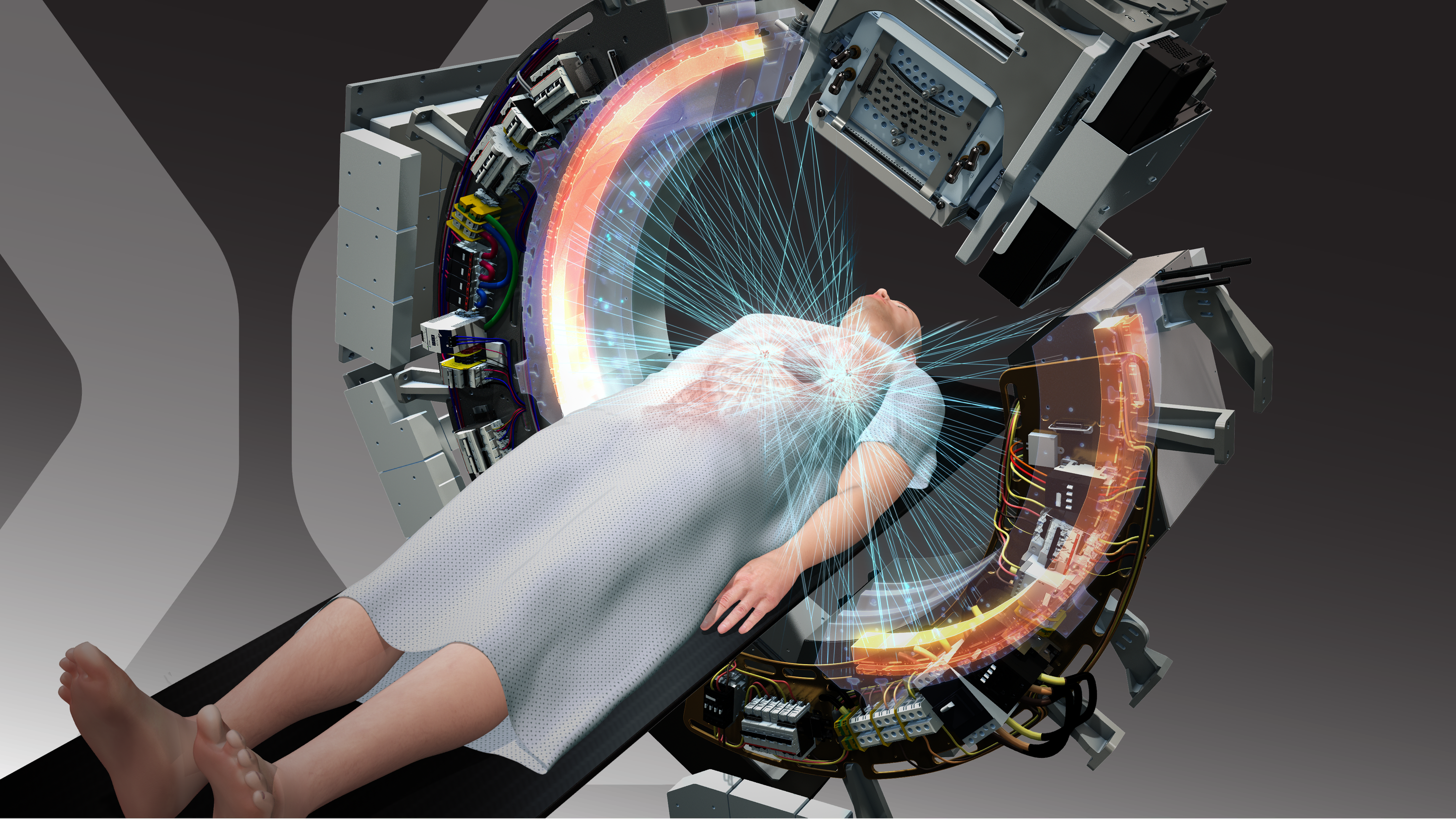
What is SCINTIX? It’s RefleXion’s trade name for biology-guided radiotherapy, a technology we developed for our X1 system, which combines a linear accelerator (LINAC) with advanced medical imaging in the form of positron emission tomography (PET) for the first time, which enables tumors to continuously signal their location. SCINTIX technology detects emissions from an injected radiotracer and, in about half a second, delivers precise doses of radiation directly to destroy cancerous tumors while sparing healthy tissue.
We already had FDA clearance to market our X1 machine for delivering conventional radiation therapy to patients with solid tumors anywhere in the body, but this new clearance applies specifically to SCINTIX for the treatment of patients with lung and bone tumors that “light up” with a particular PET tracer called FDG. These tumors may arise from primary cancers or from metastatic lesions spread from other cancers.
It was our pleasure to announce the new SCINTIX therapy clearance in a launch event at our headquarters in Hayward, Calif, on Feb. 2. In attendance were dozens of members of the RefleXion team who have worked so hard to turn science fiction into reality.
During the event, RefleXion CEO Todd Powell and founder and CTO Sam Mazin, Ph.D., reflected on how the clearance fulfills the vision of the company Mazin co-founded 15 years ago with his close friend, Akshay Nanduri and how it will impact cancer care in the years to come.
When cancer begins to spread, lung and bone are the top two locations to which it will metastasize. In fact, the clinical indications covered by the new FDA clearance represent some 400,000 patients a year in the U.S., according to Powell. Many of these patients present with stage IV disease, meaning their treatment options are limited with conventional radiation therapy.But SCINTIX therapy offers new hope to these individuals. Our technology’s ability to detect disease by using PET to measure accumulations of the radiotracer fludeoxyglucose F18 – commonly known as FDG – and then send radiation to those sites in less than a second means that cancer is basically directing its own annihilation.
“Something as seemingly impossible as making a tumor self-direct its own treatment … that’s in the realm of science fiction. It makes you feel like science fiction is just a sandbox for new technology and that humanity is capable of anything.”
Sam Mazin, PhD
CTO and Founder at RefleXion
“Something as seemingly impossible as making a tumor self-direct its own treatment … that’s in the realm of science fiction,” Mazin said during the launch event. “It makes you feel like science fiction is just a sandbox for new technology and that humanity is capable of anything.”
Powell noted the many contributions that went into moving the technology forward, ranging from advances in electromechanical technology to developments in software that were key to making SCINTIX therapy a clinical and commercial reality.
“It’s one thing to detect those signals, very, very quickly, thousands per second, and it’s an entirely different thing to make a machine that can react fast enough to actually do something therapeutically meaningful with that,” Powell said.
What’s next for RefleXion and SCINTIX? We now have clearance to market the technology to new customers who are buying X1 systems, but we will also begin rolling SCINTIX out to users in our installed base that have been employing X1 for conventional radiation therapy.
Most exciting, however, is the prospect of how our clinical partners will use the technology to advance state of the art in cancer care. RefleXion plans to work with our network of clinical partners to advance SCINTIX therapy into new areas of the body, and even to operate with new types of radiopharmaceuticals beyond FDG.
The goal of all of these initiatives is to advance cancer care to benefit individuals who haven’t been reached with conventional radiation therapy techniques.
“The way cancer is going to be treated in the next 20 years is probably going to be very different from how cancer has been treated the last 20, 40, 60 years,” Powell concluded. “It’s so exciting to dream what our clinical visionary partners will do with this technology.”
And this is only the beginning.