BgRT
RefleXion’s New Partnership Aims to Improve Prostate Cancer Treatment
November 29, 2021
Roughly one in eight men in the U.S. will be diagnosed with prostate cancer during their lifetime, making it the second most common cancer for men after skin cancer.1 Without using the recently approved molecular imaging method targeting PSMA during the initial staging, misdiagnosis of the correct prostate cancer stage happens in up to 50% of cases.2 To apply the information gathered by this new imaging and staging method to treating patients, RefleXion Medical entered into a new partnership with Lantheus Holdings, Inc.
Through the new partnership, RefleXion will investigate combining its biology-guided radiotherapy (BgRT*), with Lantheus’ PYLARIFY**, a chemical molecule for positron emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA) positive lesions. PSMA is a protein that is overexpressed on the surface of more than 90% of primary and metastatic prostate cancer cells. PYLARIFY is an approved fluorinated PET imaging agent that enables visualization of lymph nodes, bone, and soft tissue metastases to determine the presence or absence of recurrent and/or metastatic prostate cancer.
“PYLARIFY provides an accurate understanding of the location and extent of disease, which is key to creating an effective treatment plan,” said Jeffrey Wong, M.D., professor of radiation oncology at the City of Hope Cancer Center. “The potential of RefleXion’s BgRT to interpret that reliable location information and guide precise radiotherapy delivery in real-time is unprecedented. Combining BgRT’s ability to treat tumors detected by PYLARIFY both during the initial staging of patients and in biochemically recurrent prostate cancer is a powerful and promising potential advancement that may improve patient outcomes.”
“PYLARIFY allows us to potentially expand BgRT to local, recurrent and metastatic prostate cancer,” said Thorsten Melcher, Ph.D., RefleXion’s chief business officer.
Melcher explained that RefleXion’s BgRT technology has the opportunity to create a new market for PET radiotracers to provide real-time therapeutic guidance. “Lantheus has developed a novel PET imaging agent very specific for detecting local and metastatic prostate cancer. We can conceptually use that same prostate-cancer imaging agent to guide the delivery of external beam radiotherapy using the RefleXion™ X1 platform to those same prostate cancer cells,” he said.
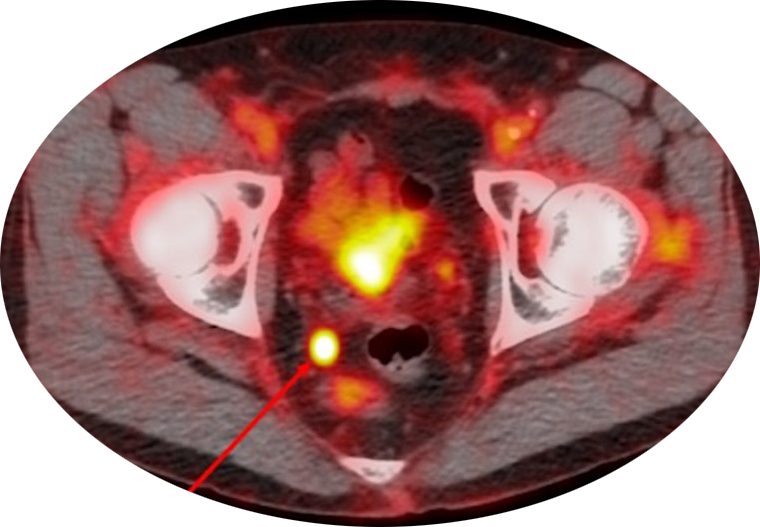
“PSMA-targeted PET tracers have increased the accuracy of correctly diagnosing the stage of prostate cancer by up to 50%.2 Mis-staging occurs because the tracing tools are less accurate and specific,” Melcher said. “In addition to metastatic prostate cancer, using a PSMA-targeted PET tracer with BgRT also potentially has relevance for treatment of local prostate cancer because clinicians may be able to tell which regions within the prostate are cancerous. PSMA is a breakthrough for prostate cancer. It also demonstrates the power of modern precision medicine.
“PYLARIFY visualizes the prostate cancer whether it’s still inside the prostate gland or if it has spread outside it to other organs,” continued Melcher. “The result will be more accurately staged prostate cancer and the ability to design the best possible treatment approach, which may be local therapy or local and systemic therapy. PYLARIFY, in combination with BgRT, can give us the most personalized prostate cancer mapping and treatment tool you could imagine.”
The RefleXion X1 machine with BgRT combines PET with a linear accelerator to guide radiotherapy delivery by using biological information created from an injected PET radiotracer. As radiotracers bind to tumors, each cancer cell produces emissions that the X1 system uses as a guide to direct radiation to each tumor.
PSMA-target PET tracers—PYLARIFY in particular—combined with BgRT represent new hope and optimism for improved diagnosis, treatment and improved outcomes for men with prostate cancer.
References
- American Cancer Society. Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
- Lawhn-Heath C, Salavati A, Behr SC, Rowe SP, Calais J, Fendler WP, Eiber M, Emmett L, Hofman MS, Hope TA. Prostate-specific Membrane Antigen PET in Prostate Cancer. Radiology. 2021 May;299(2):248-260. doi: 10.1148/radiol.2021202771.
The RefleXion™ X1 is cleared for SBRT/SRS/IMRT treatments. BgRT is limited by U.S. law to Investigational use.
** PYLARIFY (piflufolastat F-18) injection (also known as 18F-DCFPyL or PyL) received approval from the Food & Drug Administration in September 2021.