RefleXion X1
RefleXion Combination Therapy: Crossing the Divide with Biology-guided Radiotherapy to Treat the Untreatable
June 24, 2020
RefleXion Medical Inc. and Merck & Co., Inc., recently announced their clinical collaboration to evaluate the safety and efficacy of KEYTRUDA® with RefleXion’s biology-guided radiotherapy (BgRT) for treating late-stage cancers.
“We believe this exciting collaboration is among one of the first of its kind between an external-beam radiotherapy (EBRT) and a pharmaceutical company,” said Thorsten Melcher, RefleXion Medical’s chief business officer. “Increasing scientific evidence suggests that combination therapy, specifically the targeting of multiple tumors with EBRT potentiates the immunotherapy, chemotherapy or targeted therapeutic typically used to treat late-stage cancers, and thereby improves the therapeutic effect and outcome for patients.”
External-beam radiation therapy (EBRT) is the standard-of-care for patients diagnosed with cancer in stages 1, 2 or 3. “Earlier-stage cancers respond very well to EBRT, a local therapy that is effective and safe when the patient presents with only 1 to 2 sites of disease,” said Melcher. “EBRT is the most widely used ‘cancer therapeutic’ and is the standard-of-care in over 50 percent of all solid tumor patients. However, technical reasons limit how EBRT can be applied clinically.”
Patients with stage 4 cancer, also called metastatic cancer, rely on pharmaceutical drugs because currently there’s no standard-of-care using EBRT.
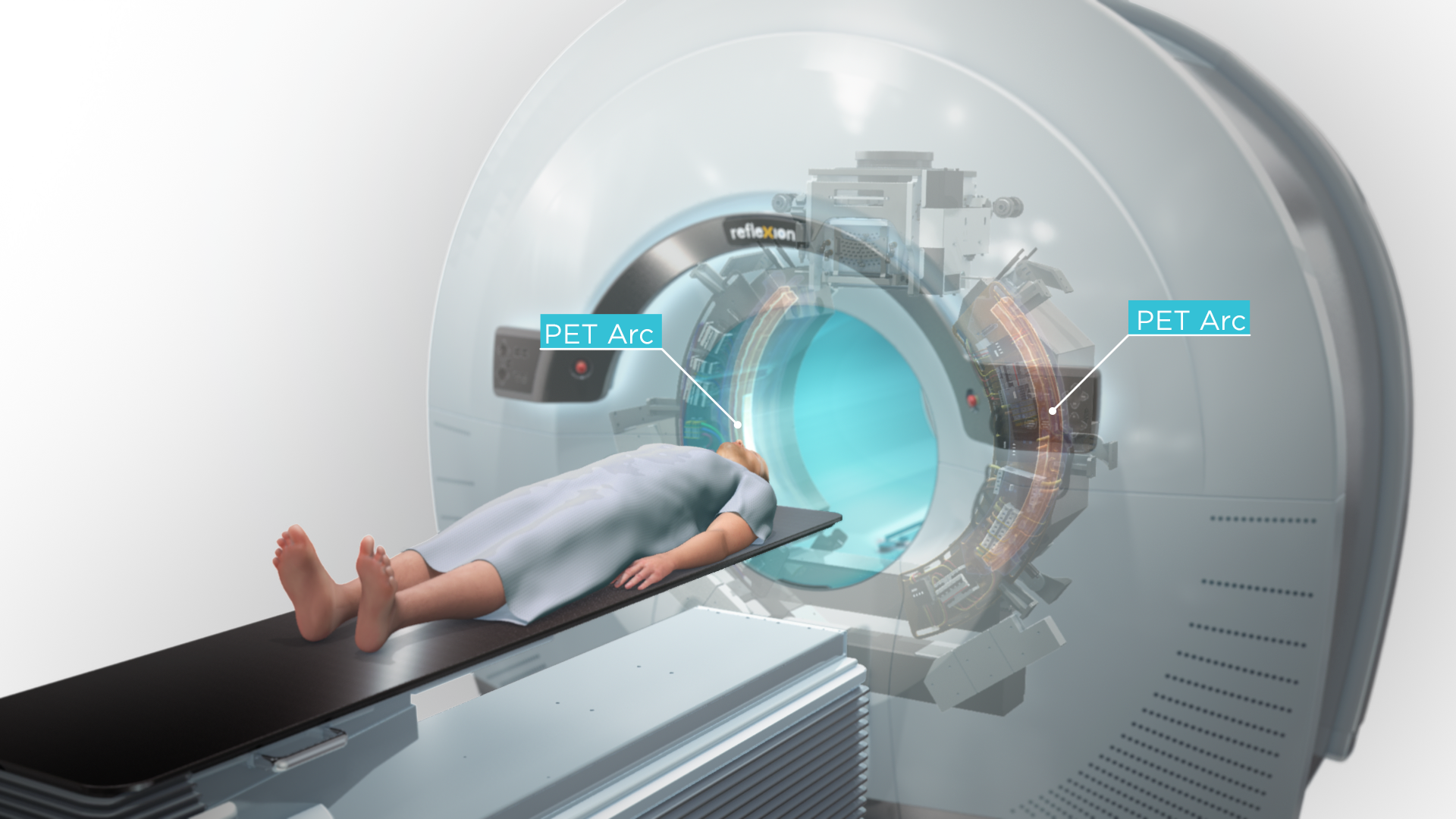
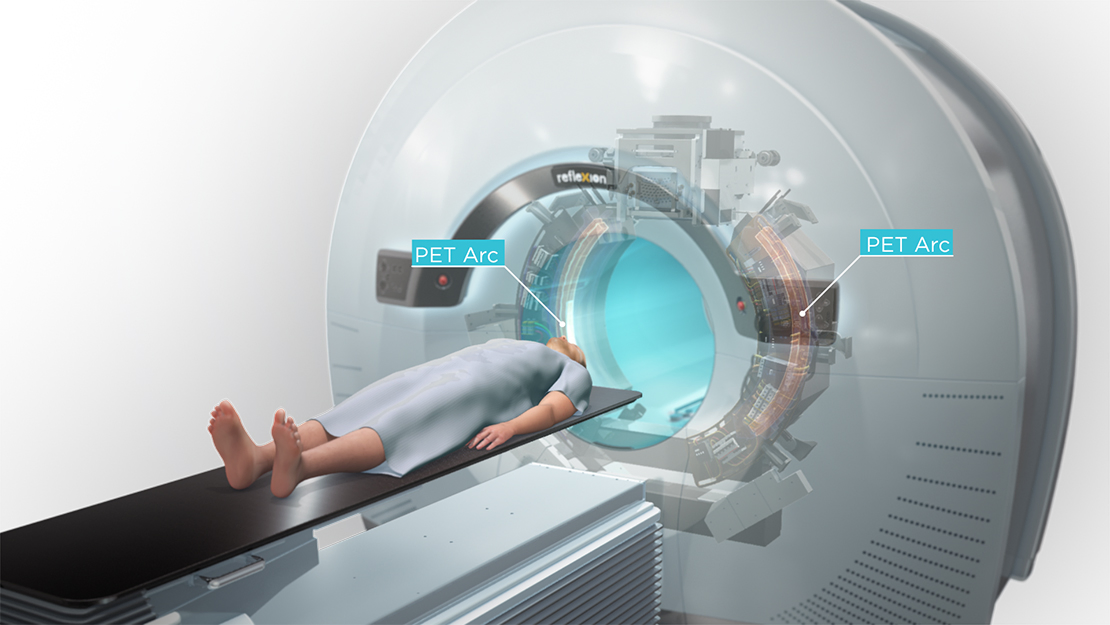
RefleXion’s BgRT is a novel advancement of EBRT that combines the functionality of PET/CT with a linear accelerator to direct radiotherapy delivery directly to tumors with sub second latency. When a radiotracer is injected into a patient, the patient’s tumors continuously signal their location—RefleXion’s X1 machine uses the signals to guide the radiotherapy beam during each fraction.
“BgRT has the potential to allow for real-time, tracked dose delivery to tumors, even in those subject to motion, in a highly parallel fashion,” said Melcher. “RefleXion’s ultimate goal is to enable treatment of many target lesions in the same session, while also sparing healthy tissue.”
RefleXion’s technology has the potential to harness two cancer treatment modalities—radiotherapy and immunotherapy—offered by medical device companies and pharmaceutical companies, respectively. Melcher explained, “RefleXion’s X1 machine is designed to overcome the technical limitations that restrict radiotherapy so it can reach multiple areas during the same treatment session—even those sites that move due to breathing or digestion.”
More than 1,000 clinical trials worldwide registered with the National Institutes of Health are focused on studying the effect of combining radiotherapy and immunotherapy. But very few of those trials are collaborations between radiotherapy and pharmaceutical companies.
The goal of RefleXion and Merck & Co’s clinical collaboration is to establish whether treating multiple tumors with BgRT is safe and amplifies Keytruda’s therapeutic effect. They will study non-small-cell lung cancer (NSCLC), the most common type of lung cancer that accounts for 84% of all lung cancer diagnoses[1]. The five-year survival rate for stage 4 NSCLC is less than 10%. Keytruda has shown significant promise in treating NSCLC. They will also study other cancers. Several factors, including accrual rates, possible modifications of the clinical protocols and choices of additional endpoints and biomarkers, will determine the duration of the clinical studies.
Earlier this year, RefleXion received FDA clearance for its X1 machine’s SBRT, SRS and IMRT functionalities. “We are actively engaged with the FDA working toward regulatory clearance for our BgRT capability. We will begin a clinical study at Stanford Cancer Institute, our first commercial site, later this year to collect clinical data in support of that application,” said Melcher.
“We want to make cancer a manageable, chronic disease, which is why this clinical collaboration with Merck & Co. is so important,” said Melcher. “The pharmaceutical industry has been very productive in creating novel immunotherapies and drugs that target certain vulnerabilities of cancer cells, and we anticipate additional collaborative opportunities in combination therapy.”
[1] https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics
*The RefleXion™ X1 is cleared for SBRT/SRS/IMRT treatments. BgRT is limited by U.S. law to Investigational use.