Telix and RefleXion Expand Partnership for Prostate Cancer Treatment
June 9, 2022
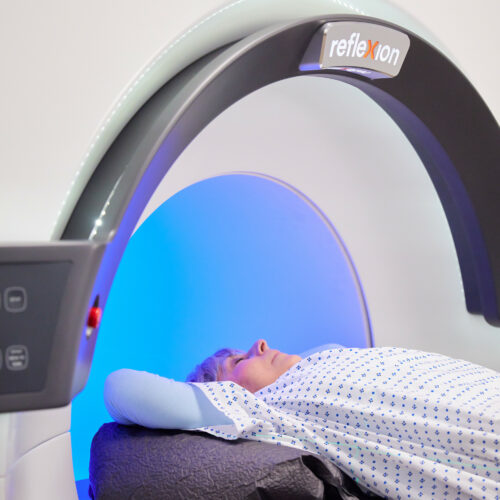
Melbourne (Australia) and Indianapolis, IN (U.S.) – 9 June 2022. Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) and RefleXion Medical, Inc. (Hayward, California, U.S.) (RefleXion) today announced the signing of a co-development and commercialisation agreement, to expand the use of Telix’s prostate cancer imaging agent, Illuccix® (kit for preparation of gallium (68Ga) gozetotide) with the RefleXion® biology-guided radiotherapy (BgRT)* platform to guide external-beam radiotherapy in real-time.
BgRT is the first and only cancer treatment designed to integrate PET technology as part of external-beam radiotherapy delivery. It uses PET tracers as biological guides to signal the location of cancer and guide the delivery of radiotherapy to tumours in real-time. BgRT has the potential to offer significant advantages over conventional radiotherapy as it may one day enable treatment of multiple tumors per session for metastatic disease, increase the conformality of radiotherapy delivery, and reduce toxicity to healthy tissue. This approach may facilitate treatment of later stage cancers than is currently practical for hospitals or tolerable by patients.
Under the agreement, which builds on an existing strategic collaboration1 between the companies, Telix and RefleXion will conduct and co-fund a BgRT clinical program using Illuccix as a biological guide, seek regulatory approval and jointly pursue commercialization, initially in the United States. The parties will share in any upside generated if successfully commercialized. The clinical program is expected to commence in 2023. RefleXion is exclusively partnering with Telix for 68Ga PSMA-PET imaging agents for use with BgRT. The agreement also includes the potential to expand the exclusive relationship beyond the U.S., in countries where both Illuccix and BgRT are intending to be commercialised.
More than 60,000 men undergo external-beam radiotherapy for prostate cancer every year in the U.S. alone2.
If approved, Illuccix for BgRT could potentially open a broad new market opportunity for Illuccix as a therapy guidance agent. More than 60,000 men undergo external-beam radiotherapy for prostate cancer every year in the U.S. alone2. Illuccix for BgRT would require up to five doses per patient (one for each session of external-beam radiotherapy), potentially expanding the volume of Illuccix used in the U.S. significantly. The scheduling flexibility of Gallium-based compounds such as Illuccix may provide additional advantages for patients and caregivers using BgRT technology.
Dr. Christian Behrenbruch, Group CEO of Telix Pharmaceuticals said, “Following a successful evaluation period, we are pleased to expand our relationship with RefleXion and move ahead with a clinical program with the objective of regulatory approval of Illuccix for BgRT. This partnership demonstrates the potential for Illuccix and other molecularly-targeted imaging agents in our pipeline to be used as a tool to both detect the presence of metastatic disease and guide treatment using innovative complementary technologies such as BgRT.”
“With PSMA-PET quickly being established as a standard of care in prostate cancer imaging, the clinical program aims to determine whether Telix’s gallium-based tracer can provide a complete and robust signal to guide BgRT to treat all stages of prostate cancer, eventually including metastatic disease.”
Dr. Thorsten Melcher
Chief Business Officer at RefleXion
Dr. Thorsten Melcher, Chief Business Officer at RefleXion added, “With PSMA-PET quickly being established as a standard of care in prostate cancer imaging, the clinical program aims to determine whether Telix’s gallium-based tracer can provide a complete and robust signal to guide BgRT to treat all stages of prostate cancer, eventually including metastatic disease. Illuccix offers great accessibility and scheduling flexibility, factors which we believe will be well-suited to the treatment regimen with our BgRT system.”
Reflexion has received marketing clearance from the United States Food and Drug Administration (FDA) for its X1 machine that combines high quality computed tomography (CT) imaging with stereotactic body radiotherapy (SBRT), stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT).
About RefleXion Medical
RefleXion is a privately-held company developing the first biology-guided radiotherapy system, a significant change in strategy from single tumor therapy to the ability to one day treat multiple tumors in the same treatment session in cancers that have metastasized. Currently, the RefleXion X1 machine is cleared for the delivery of stereotactic body radiotherapy (SBRT), stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT). The company is also developing BgRT, which incorporates positron-emission tomography (PET) imaging data to enable tumors to continuously signal their location. The BgRT technology will synchronize these data with the linear accelerator to direct radiotherapy to tumors with sub-second latency. For more information, visit www.reflexion.com and follow RefleXion on Twitter (@reflexionmed) and LinkedIn.
*The RefleXion® X1 is cleared for SBRT/SRS/IMRT. BgRT is pending regulatory review and is not commercially available.
About Telix Pharmaceuticals Limited
Telix is a biopharmaceutical company focused on the development and commercialisation of diagnostic and therapeutic products using Molecularly Targeted Radiation (MTR). Telix is headquartered in Melbourne, Australia with international operations in Belgium, Japan, Switzerland, and the United States. Telix is developing a portfolio of clinical-stage products that address significant unmet medical need in oncology and rare diseases. Telix is listed on the Australian Securities Exchange (ASX: TLX). For more information visit www.telixpharma.com and follow Telix on Twitter (@TelixPharma) and LinkedIn.
RefleXion Media Contact
Amy Cook
RefleXion Medical
Email: acook@reflexion.com
Telix Investor Relations
Ms. Kyahn Williamson
Telix Pharmaceuticals Limited
SVP Corporate Communications and Investor Relations
Email: kyahn.williamson@telixpharma.com
1 ASX release 8 July 2020
2 https://www.pcf.org/c/treatment-for-prostate-cancer-external-beam-radiation-therapy/