Abstracts
“20 in 2020”
January 28, 2021
The efficacy of any new cancer treatment, be it technology-based or pharmaceutical, depends on the strength and viability of the evidence-based research that supports it. Extensive clinical research, analysis and peer review are necessary precursors to approval and ultimate use. RefleXion Medical continues to expand its evidence base. In the past 12 months, 20 abstracts have been published validating the promise of this new technology.
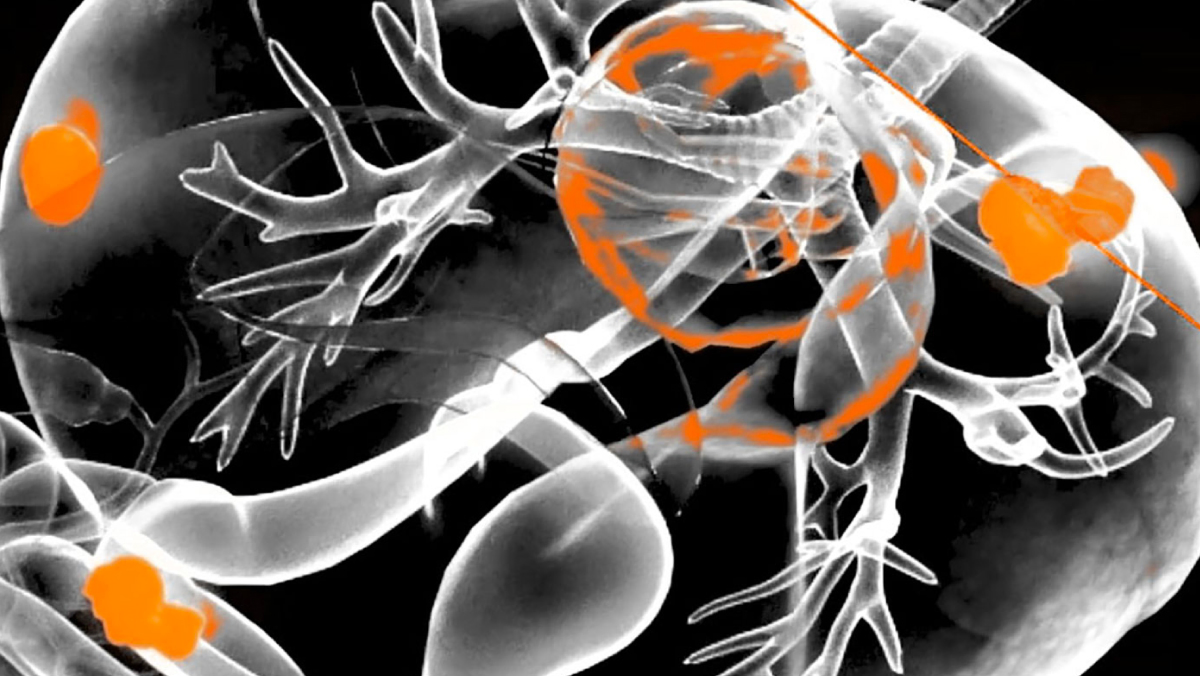
Our RefleXion X1 platform is designed from the ground up to use PET emissions to deliver tracked radiation dose in real-time, which we believe will deliver a more conformal dose and decrease exposure to normal tissue. These technological achievements are verified by meeting or exceeding industry standards set by the American Association of Physicists in Medicine (AAPM) and supported through academic research. We presented seven of the 20 abstracts produced in 2020 at the AAPM annual meeting, demonstrating that the RefleXion X1 platform that will enable BgRT meets or exceeds AAPM standards for quality assurance in dose delivery, treatment planning and image quality.
At the 2020 American Society for Radiation Oncology (ASTRO) annual meeting last October, we presented eight abstracts to clinicians on the emulated use of BgRT in the following:
- dose escalation to targets,
- treatment of pancreatic tumors,
- treatment of metastatic prostate cancer, and
- as a prognostic tool for metabolic tumor volume assessment.
Additionally, our prototype planning system was compared to standard VMAT and HT planning for head and neck radiotherapy by clinical scientists from a leading academic medical center that found our prototype SBRT plans achieved comparable PTV dose coverage and lower dose to most critical organs, including a significant reduction in parotid mean dose compared to the standard VMAT and HT plans. This research was published in Medical Dosimetry in November 2020.
Research involving BgRT technology was also presented at the 2020 European Society for Therapeutic Radiation Oncology (ESTRO) annual meeting, exhibiting the feasibility of BgRT in the treatment of metastatic cancer.
At the same time, an entirely new avenue for research and clinical tools is opening to physicians via radiomics and the RefleXion X1 platform. As a result, physicians will one day be able to understand how tumors change during the course of treatment with the use of PET imaging by analyzing tumor characteristics such as total metabolic tumor volume (MTV) and/or total lesion glycolysis (TLG) assessments.
The resulting PET and CT images that BgRT produces as a byproduct of each treatment fraction provide a vast amount of imaging data for analysis that may one day evolve into the creation of biomarkers to help predict treatment response and overall survival in patients with specific types of cancer. This new information may help physicians in clinical decision-making regarding treatment management.
Two abstracts presented at the 2020 ASTRO meeting addressed the use of PET for this purpose: The prognostic value of FDG-PET metrics for advanced Non-Small Cell Lung Cancer (NSCLC) patients treated with first-line immunotherapy; and FDG-PET metrics in advanced NSCLC: A systematic review and meta-analysis.
The first abstract reported clinicians’ exploration of the prognostic value of FDG-PET for NSCLC patients treated with first-line immunotherapy and whether PET-guided radiotherapy (RT) should be explored in those patients. The analysis included 72 patients treated between 2015 and 2019 with first-line immune checkpoint inhibitors targeting the PD-1/PD-L1 axis (with or without chemotherapy) for advanced/metastatic NSCLC who underwent PET staging. Researchers tested the number of hypermetabolic lesions (nLesions) and total metabolic tumor volume (MTV, log-transformed) as predictors of progression-free survival (PFS) and overall survival (OS) duration. Among patients who received RT near the time of diagnosis, the proportion of disease treated with RT was also tested as a prognostic factor. Researchers found that PET-based measures of disease burden (MTV) and multifocality (nLesions) may be important prognostic factors for advanced NSCLC patients treated with first-line immunotherapy. They also determined that disease ‘debulking’ with RT could be explored as an adjunct to immunotherapy.
The second abstract reported results from a systematic review and meta-analysis of published literature characterizing the prognostic value of pre-treatment, volume-based FDG-PET metrics in patients with advanced NSCLC. Researchers identified studies describing the prognostic value of volume-based PET metrics (total metabolic tumor volume [MTV] and/or total lesion glycolysis [TLG]) obtained prior to initiation of first-line systemic therapy for advanced—at least 50% Stage IV) NSCLC. Progression-free survival (PFS) and overall survival (OS) were the progression points examined. The study authors extracted hazard ratios for PFS and OS directly from the original reports when available or estimated from survival curves using customized scripts. They performed inverse variance meta-analyses to assess associations between PET metrics and clinical outcomes. The analysis included 12 articles comprising 984 patients, and the percentage of patients with Stage IV disease ranged from 67% to 100%. Patients from at least nine studies received chemotherapy and patients from at least four studies were treated with targeted therapy. No studies examining PET metrics for patients treated with first-line immunotherapy were identified. Researchers concluded that baseline PET metrics (MTV, TLG) are powerful prognostic factors for advanced NSCLC patients who are treated with chemotherapy or targeted therapy.
Looking ahead, technological research coupled with continued epidemiologic validation and clinical use of BgRT will be at the forefront of our continued work as we strive to one day offer a new treatment modality for patients with all stages of cancer, especially those with metastatic disease.
*The RefleXion™ X1 is cleared for SBRT/SRS/IMRT treatments. BgRT is limited by U.S. law to Investigational use.